
A volunteer sits in a doctor’s office in Binghamton, New York. She’s wearing a sundress, so there’s no need to roll up a sleeve. A researcher walks in and gives her a shot in the upper arm. “Well, that was painless,” the volunteer says, cheerfully.
That was July 27, the day the first potential coronavirus vaccine reached late-stage testing in the United States. It was developed by a company called Moderna and the National Institutes of Health. Human trials
trial
 AMPHOTORA—GETTY IMAGES
a test of the quality, value, or usefulness of something
(noun)
A clinical trial showed that the drug was safe and helpful.
began in March. Tens of thousands of volunteers would sign up. Now, two more companies have reached late-stage vaccine testing in the U.S.
AMPHOTORA—GETTY IMAGES
a test of the quality, value, or usefulness of something
(noun)
A clinical trial showed that the drug was safe and helpful.
began in March. Tens of thousands of volunteers would sign up. Now, two more companies have reached late-stage vaccine testing in the U.S.

VACCINE TESTING Some vaccine trials are taking place at the Research Centers of America, in Hollywood, Florida.
CHANDAN KHANNA—AFP/GETTY IMAGESA vaccine would protect people against the virus that causes COVID-19. It would save lives and help end the pandemic. With enough people inoculated
inoculate
 FATCAMERA—GETTY IMAGES
to protect from disease
(verb)
We took our dog to the veterinarian so she could be inoculated against rabies.
, schools and businesses could fully reopen. That’s why there’s such a rush to develop a vaccine.
FATCAMERA—GETTY IMAGES
to protect from disease
(verb)
We took our dog to the veterinarian so she could be inoculated against rabies.
, schools and businesses could fully reopen. That’s why there’s such a rush to develop a vaccine.
Need for Speed
A vaccine works by building the body’s immunity
immunity
 CHOJA—GETTY IMAGES
freedom from sickness or harm
(noun)
Because I had chickenpox last year, I now have immunity from it.
to a virus. “It teaches the body to recognize the virus,” Dr. Rick Malley, of Boston Children’s Hospital, in Massachusetts, told TIME for Kids. “The next time the body sees the bug, it fights it off without ever getting sick.”
CHOJA—GETTY IMAGES
freedom from sickness or harm
(noun)
Because I had chickenpox last year, I now have immunity from it.
to a virus. “It teaches the body to recognize the virus,” Dr. Rick Malley, of Boston Children’s Hospital, in Massachusetts, told TIME for Kids. “The next time the body sees the bug, it fights it off without ever getting sick.”
It can take 10 years to develop a vaccine. Scientists have to do research and produce a vaccine they can test (see “Road to Success”). It must pass three phases of human trials. Only then can it be approved, manufactured, and distributed.
This process has been made faster by Operation Warp Speed. The U.S. government launched the program in May. It gives billions of dollars to companies so they can quickly make coronavirus vaccines.
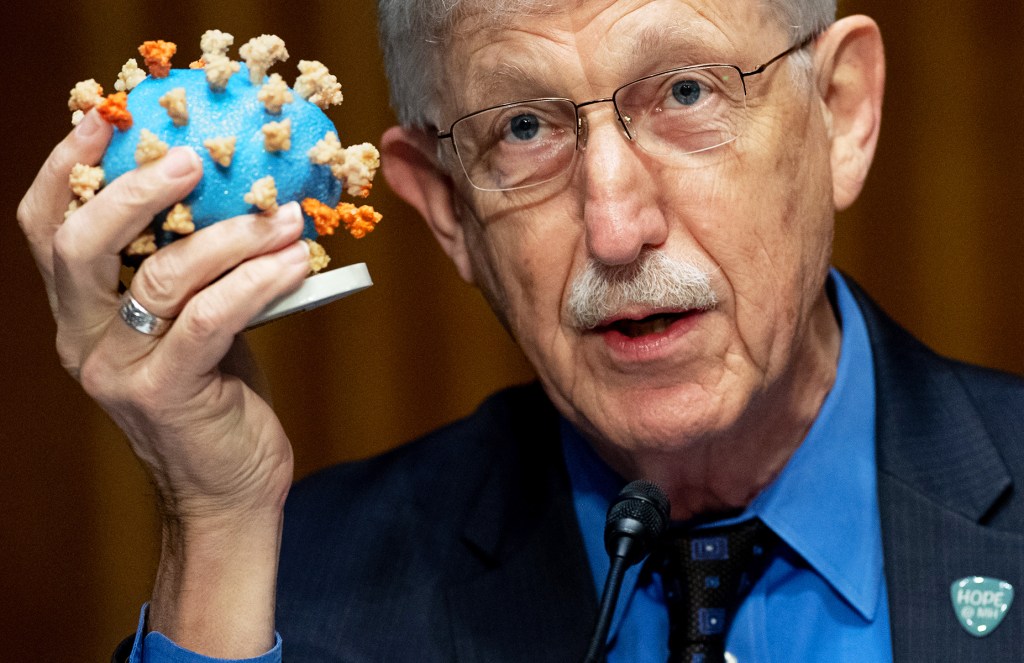
FIGHTING THE VIRUS Dr. Francis Collins, of the National Institutes of Health, speaks about Operation Warp Speed in Washington, D.C., on July 2.
SAUL LOEB—AFP/BLOOMBERG/GETTY IMAGESSome companies have used money from Operation Warp Speed to manufacture doses of a vaccine before the final phase of testing is complete. If it’s safe and effective, the vaccine can then be distributed right away.
Down the Line
It’s still uncertain when a vaccine will be available in the U.S. Robert Redfield is director of the Centers for Disease Control and Prevention. On September 2, he told Yahoo Finance that “one or more vaccines” could be ready by the end of the year.
Moncef Slaoui is a scientist and one of the leaders of Operation Warp Speed. On August 31, he told National Public Radio that “we may have enough vaccine by the end of the year [for] between 20 [million] and 25 million people.”
That means many people in the U.S. will have to wait. The first doses will likely go to health-care workers and elderly people. “We have to figure out how to make sure they’re distributed in a fair and equitable
equitable
 FLORESCO PRODUCTIONS/GETTY IMAGES
fair and equal
(adjective)
Teachers should have an equitable system for giving students detention.
way,” Redfield said.
FLORESCO PRODUCTIONS/GETTY IMAGES
fair and equal
(adjective)
Teachers should have an equitable system for giving students detention.
way,” Redfield said.
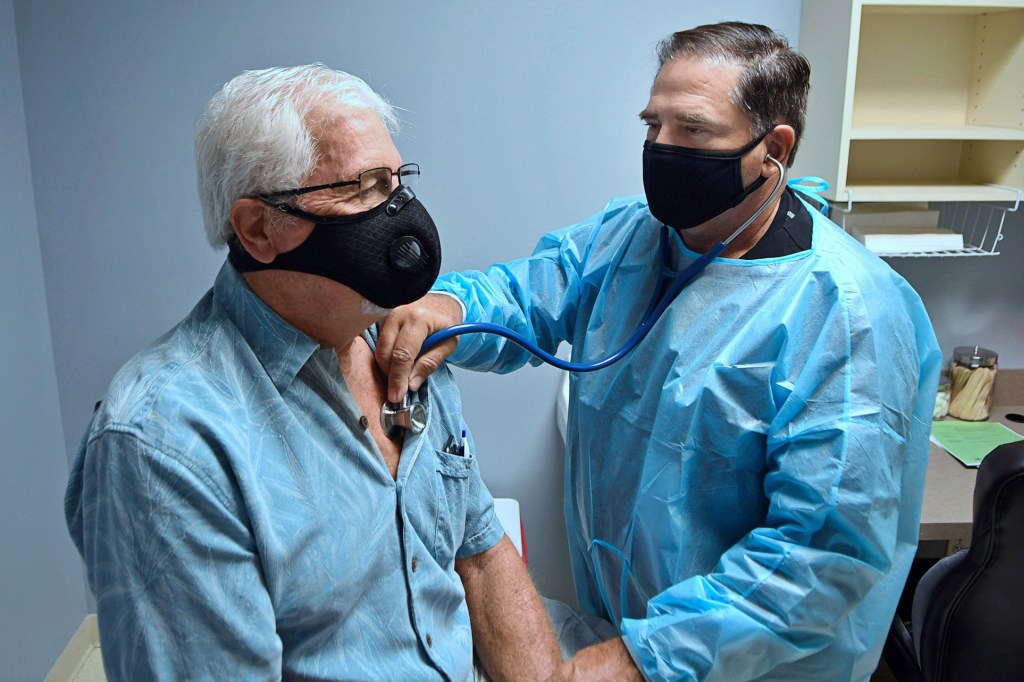
PUT TO THE TEST On August 4, a volunteer is examined before participating in a coronavirus vaccine trial in DeLand, Florida.
PAUL HENNESSY—NURPHOTO/GETTY IMAGESOther countries are working on vaccines too. Australia, China, India, Italy, Japan, Singapore, and South Korea are in the human-trials stages. And on August 11, Russian president Vladimir Putin announced that his country had approved a coronavirus vaccine. He called it a “world first.”
As the U.S. races toward a vaccine, safety is a top concern. On September 8, the leaders of nine companies working on vaccines in the U.S. issued a statement: They will not release a vaccine before they’re sure it’s safe. The companies promise to make “the safety and well-being of vaccinated individuals our top priority.”
Road to Success
A lot of work goes into making a new vaccine. Here are the five steps.
Before trials: Scientists conduct research and run preliminary tests on cells and animals.
Phase 1: The vaccine is tested on a small group of human volunteers to make sure it’s safe.
Phase 2: The vaccine is tested on a slightly larger group of volunteers, again to check for safety.
Phase 3: The vaccine is tested on thousands of volunteers. Researchers find out if the vaccine is effective.
After trials: A vaccine may now be approved by the government. It can then be manufactured and distributed to the public.













